Tag: Healthcare%20as2
October 16, 2023
Communicating with Tatmeen for UAE Regulation Compliance
Tatmeen is a digital platform developed by the UAE’s Ministry of Health and Prevention that enables the tracking and tracing of all pharmaceutical products in the UAE. Tatmeen regulations are already in effect, so it is important to prioritize B2B integration solutions that are easy to use and quick to set up. Communicating with Tatmeen becomes much easier with AS4 communication. CData Arc has been providing Drummond-certified AS4 integration technologies for over a decade, making it a trusted tool for AS4 implementation. [read more]
October 12, 2023
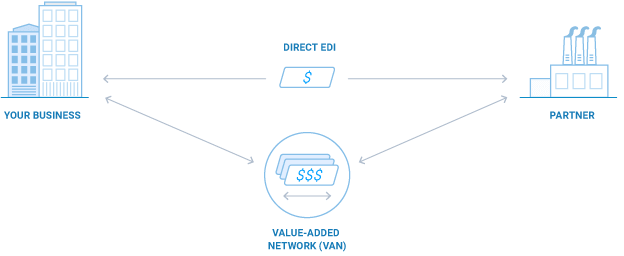
Direct EDI (AS2) vs. VANs: Pros, Cons and The Basics
Throughout the world, companies large and small alike are increasingly adding, expanding, and modernizing their EDI communications. If you need to meet partner EDI mandates or wish to capture the many benefits afforded by EDI connectivity with more of your partners, you can take several approaches ... [read more]
December 16, 2022
CData Arc Again Receives AS2 and AS2 Cloud Drummond Certification
CData Arc has once again received AS2 and AS2 Cloud Interoperability Certification from Drummond. [read more]
January 17, 2022
CData Arc Successfully Completes AS2 and AS2 Cloud Drummond Certification
CData Arc received its 20th AS2 and AS2 Cloud Interoperability Certification from Drummond in December 2021. Drummond is the gold standard for B2B interoperability conformance testing, with over 40,000 tests performed to receive certification. What is Interoperability Testing and Why is it Importan... [read more]
June 21, 2021
Integrating Regulatory Submissions with the FDA Electronic Submissions Gateway (ESG)
If your food or pharmaceutical products require approval from the Food and Drug Administration (FDA), you'll need to submit them through the FDA's Electronic Submissions Gateway, commonly known as the FDA ESG. The FDA streamlines their communications through electronic exchange of text and data, pr... [read more]
June 11, 2021
Electronic Data Interchange (EDI) in Healthcare with CData Arc
When it comes to exchanging healthcare data, whether for medical treatments or health insurance reimbursements, security and confidentiality are essential. That's why Congress passed the Health Insurance Portability and Accountability Act (HIPAA) in 1996 and strengthened privacy and security for el... [read more]
June 09, 2021
FHIR: Modernized Healthcare EDI
Enterprise organizations constantly push for ways to modernize and streamline their data movement. EDI standards (e.g. ANSI X12, HL7) have long been the dominant paradigm for data modeling and communication within the healthcare industry, but a new data standard developed by HL7 International has a... [read more]
August 24, 2020
What is Healthcare EDI Validation? And How to Get Started.
SNIP Validation ensures healthcare EDI documents, such as an X12 HIPAA 834 Benefit Enrollment or an X12 HIPAA 837 Healthcare Claim, are correctly formatted to adhere to the schemas defined in the X12 HIPAA EDI standard. SNIP Validation uses seven levels, or types — SNIP Type 1 is the least strict a... [read more]
July 02, 2020
How to Set Up EDI with Amazon Vendor Central
An invitation to the Amazon Vendor Central platform is a crossroads, providing a coveted opportunity to boost sales and reach scale. The challenge is complying with Amazon's stringent trading requirements, which force organizations to move into the deep waters of EDI . In this article, we cover the... [read more]